使用乳牙牙髓干细胞培养上清经特殊加工制成的99.9%纯Exosome静脉滴注疗法
Exosome是什么
Exosome是指体内存在的50到150纳米大小的非常小的脂质包膜小囊泡。
Exosome中含有微小RNA、信使RNA、DNA、蛋白质等物质,其中微小RNA和信使RNA在细胞之间进行重要的信息传递,这是近年来被确认的细胞间重要的通信工具。
通过利用Exosome的这些特性进行治疗,可以在各个领域,包括抗衰老护理等方面发挥效果。
我们医院使用Exosome进行治疗
Exosome的潜力巨大,以各种方式影响人体的37万亿个细胞,对细胞变化和日常生命的维持起着重要作用。
目前,我们正在推动乳牙牙髓干细胞来源的Exosome治疗和数据分析在相关医疗机构的发展。
PURE EXO是什么
PURE EXO是从乳牙牙髓干细胞中提取的Exosome,以99.9%的纯度精制而成。相比于培养上清液,培养上清液在将Exosome引入体内时需要投入大量的量,而PURE EXO可以以大约1/10的量将大约是培养上清液的10倍量的Exosome引入体内。
培养上清液中除了包含Exosome外,还包含各种生长因子、细胞因子和培养液。由于PURE EXO只分离了Exosome,因此可以在不引发细胞因子风暴的情况下进行大规模的Exosome注射。
SGF 5毫升中含有100亿个Exosome,而PURE EXO可以在一次注射中引入1,000-2,000亿个Exosome。
在SGF中,可以一次注射5毫升至20-30毫升(Exosome 100亿至500亿个),但要引入1,000亿个以上的Exosome,必须使用PURE EXO。
适合以下这些人
期待效果
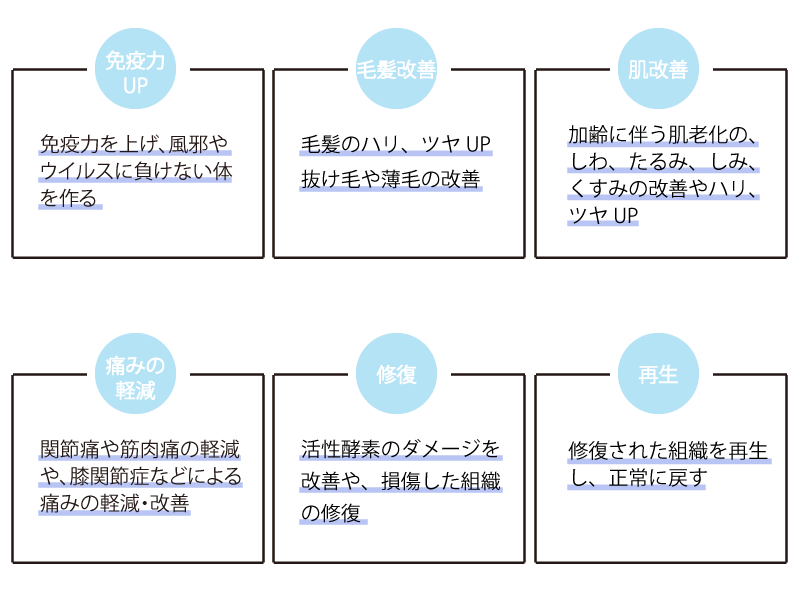
Re Clinic Ginza使用Exosome进行的治疗和成果
1.
• 预防和治疗动脉硬化等缺血性疾病
• 改善血管内皮障害
• 预防和改善糖尿病等慢性内科疾病
• 对端粒延长的影响
【治疗方法】静脉注射和静脉滴注
2.
• 男性勃起功能障碍(ED)
• 雄性秃疮(AGA)
• 缓解肘膝关节疼痛等关节疾病
• 男性不育治疗
• 皮肤质量改善
【治疗方法】局部注射
3.
• 认知症的改善和预防
• 脑卒中后遗症(半身瘫痪等)的改善
• 阿尔茨海默病型认知症
• 躁郁症等精神疾病
【治疗方法】鼻腔点滴
4.
• 视力改善
料金表
| PURE EXO 100(Exosome100亿个) | 110,000日元 |
| PURE EXO 1000(Exosome1000亿个) | 1,100,000日元 |
Exosome的结构
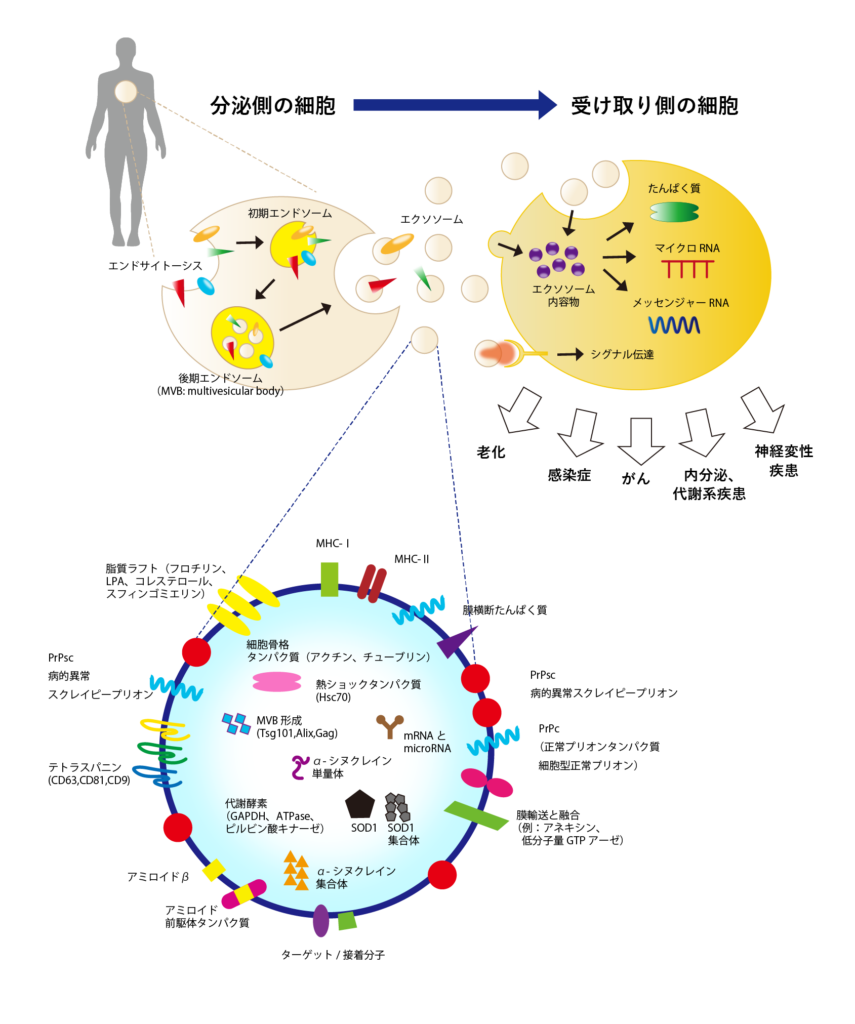
Exosome是通过内吞作用(细胞内部取入外部物质的过程)形成的内膜小泡,然后通过释放到细胞外而产生的。Exosome内部包含原始细胞的物质,表面有膜蛋白如四膜蛋白家族(如CD9、CD63、CD81等)和整合素等,以及内部含有与多囊体形成相关的蛋白质(Tsg101、Alix)和热休克蛋白(HSP)。
Exosome是用于细胞间信息传递的信使
Exosome被释放出来自细胞,通过血液流动在全身。在这个过程中,Exosome中包含的微小RNA和信使RNA与体内各个器官的细胞进行通信,并引发各种生理反应。比如,它可能传达信息,要求加快新陈代谢或形成新的血管。
这种通信不仅发生在不同器官之间,如“从肠细胞到脑细胞”或“从肾脏细胞到肠细胞”,而且还需要Exosome作为信息的使者。以前的传统观念认为,所有这些通信都是通过大脑进行的。然而,通过Exosome的研究,我们发现事情并非如此。
例如,当我们吃得过多时,身体会自然地增加代谢以消耗食物,这表明我们之所以能够保持健康的生活方式,是因为各个器官互相合作,以维持良好的状态。
Exosome和其他细胞之间直接进行通信,以协调各个器官的功能,从而使我们的身体正常工作,保持健康状态。
幹细胞培养上清液与幹细胞Exosome的区别
“幹细胞培养上清液和Exosome是不同的”
干细胞培养上清液的制备方法和质量控制非常重要。质量应该通过检查干细胞Exosome的纯度和标志物(CD9、CD63)来确认,而不是通过粒子总数。除了干细胞Exosome之外的颗粒可能对身体有害。
期望从间充质干细胞Exosome中获得的效果
已经有许多研究者在研究间充质干细胞(MSC)分泌的Exosome在医疗应用等方面的潜力,并已经报道了治疗效果。
据文献报道,间充质干细胞来源的Exosome已经显示出与干细胞本身相当或更多的效果。
Expert Opin Biol Ther. 2016;16(4):489-506. doi: 10.1517/14712598.2016.1131976. Epub 2016 Jan 28.
Exosomes for repair, regeneration and rejuvenation Joydeep Basu, John W Ludlow
脂肪細胞の血管新生促進の促進 促进脂肪细胞血管新生的研究
在使用小鼠进行的研究中,比较了脂肪干细胞Exosome、经处理的脂肪干细胞以及仅使用生理食盐水的脂肪细胞(组织)的定植情况。
研究发现,与仅使用生理食盐水的情况相比,脂肪干细胞Exosome和经处理的脂肪干细胞在血管新生和定植水平上存在明显差异。此外,在比较Exosome和脂肪细胞的情况时,Exosome的效果与使用脂肪细胞时的定植效果相当。
这表明Exosome的效果与干细胞移植具有相等的效果证据。
Plast Reconstr Surg. 2019 Nov;144(5):816e-827e. doi: 10.1097/PRS.0000000000006175.
Exosomes Are Comparable to Source Adipose Stem Cells in Fat Graft Retention with Up-Regulating Early Inflammation and Angiogenesis
Bin Chen, Junrong Cai, Yating Wei, Zhaohua Jiang, Haley E Desjardins, Alexandra E Adams, Shengli Li, Huang-Kai Kao, Lifei Guo
关于间充质干细胞Exosome对细胞和组织修复、再生和抗衰老的研究
通过以下研究论文,已经对间充质干细胞Exosome在细胞和组织修复、再生以及抗衰老领域进行了广泛研究,并取得了许多研究成果。
Expert Opin Biol Ther. 2016;16(4):489-506. doi: 10.1517/14712598.2016.1131976. Epub 2016 Jan 28.
Exosomes for repair, regeneration and rejuvenation Joydeep Basu, John W Ludlow
3. Maguire G. Stem cell therapy without the cells. Commun Integr Biol. 2013;6:e26631. DOI:10.4161/cib.26631.
5. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15.
13. Valadi H, Ekstrom K, Bossios A, et al. Exosome mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 2007;9:654-659. Demonstration of exosome-mediated communication between cells during development
14. Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell derived micro-vesicles reprogram hematopoietic pro-genitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-856.
19. Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological proper-ties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4. DOI:10.3402/jev.v4.27066.
32. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizon-tal transfer of mRNA. Blood. 2007;110:2440-2448.
40. Teixeira FG, Carvalho MM, Sousa N, et al. Mesenchymal stem cells secretome: a new paradigm for central ner-vous system regeneration. Cell Mol Life Sci. 2013;70:3871-3882.
41. Vishnubhatla I, Corteling R, Stevanato L, et al. The devel-opment of stem cell-derived exosomes as a cell-free regenerative medicine. J Circ Biomark. 2014;3:2. DOI:10.5772/58597.
43. Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2013;9:1045-1059.
44. van Koppen A, Joles JA, van Balkom BW, et al.ヒト胚性間葉系幹細胞由来の馴化培地は、確立された慢性腎臓病のラットの腎機能を救います。 PLoS ONE。
(?)onescu L、Byrne RN、van Haaften T、他。幹細胞馴化培地は、マウスの急性肺損傷を改善します: 幹細胞パラクリン作用の in vivo 証拠。
Am J Physiol 肺細胞 Mol Physiol。 2012;303:L967–977.
45. Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strate-gies. Leukemia. 2012;26:1166-1173.
50. Osugi M, Katagiri W, Yoshimi R, et al. Conditioned media from mesenchymal stem cells enhanced bone regenera-tion in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479-1489.
51. Maumus M, Jorgensen C, Noel D. Mesenchymal stem cells in regenerative medicine applied to rheumatic dis-eases: role of secretome and exosomes. Biochimie. 2013;95:2229-2234.
52. Toh WS, Foldager CB, Pei M, et al. Advances in mesench-ymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Reviews and Reports. 2014;10:686-696.
53. Akyurekli C, Le Y, Richardson RB, et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11:150-160. Review of key preclinical studies associated with mesenchymal stem cell (MSC)-sourced exosomes
55. Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells pro-tect against ischaemia reperfusion induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474-1483.
56. Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotec-tive miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61-69.
57. Kilpinen L, Impola U, Sankkila L, et al. Extracellular membrane vesicles from umbilical cord blood derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;10:3402.
60. Lamichhane TN, Sokic S, Schardt JS, et al. Emerging roles for extracellular vesicles in tissue engineering and regen-erative medicine. Tissue Eng Part B Rev. 2015;21:45-54. DOI:10.1089/ten.teb.2014.0300.
61. Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724-728.
62. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia induced pulmonary hypertension. Circulation. 2012;126:2601-2611.
63. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells derived MSCs facilitate cutaneous wound healing by promoting col-lagen synthesis and angiogenesis. J Trans Med. 2015;13:49. DOI:10.1186/s12967-015-0417-0.
64. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-619.
71. Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expres-sion. Stem Cells Dev. 2014;23:2851-2861.
73. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome-encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-1779. DOI:10.1038/mt.2011.164.
74. Xin H, Li Y, Liu Z, et al. Systematic administration of exo-somes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711-1715.
75. Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856-867.
76. Doeppner TR, Herz J, Gorgens A, et al. Extracellular vesi-cles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143.
77. Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plas-ticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-2746.
78. Bruce A, Ilagan R, Guthrie K, et al. Selected renal cells modulate disease progression in rodent models of chronic kidney disease via NFκB and TGFβ1 pathways. Regen Med. 2015;10:815-839.
79. Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. DOI:10.3389/fimmu.2014.00370.
82. Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological func-tions, and diagnostic and therapeutic potentials. Biochimica et Biophysica Acta. 2012;1820:940-948.
83. Kordelas L, Rebmann V, Ludwig A-K, et al. 2013. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-973. DOI:10.1038/leu.2014.41.
101. Lee HJ, Lee EG, Kang S, et al. Efficacy of microneedling plus human stem cell conditioned medium for skin reju-venation: a randomized, controlled, blinded split face study. Ann Dermatol. 2014;26:584-591.
102. Harn HJ, Huang MH, Huang CT, et al. Rejuvenation of aged pig facial skin by transplanting allogeneic granulo-cyte colony stimulating factor induced peripheral blood stem cells from a young pig. Cell Transplant. 2013;22: 755-765.
103. Kim WS, Park BS, Park SH, et al. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96-102.
104. Shim JH, Park JY, Lee MG, et al. Human dermal stem/ progenitor cell-derived conditioned medium ameliorates ultraviolet a induced damage of normal human dermal fibroblasts. PLoS One 2013;e67604. DOI:10.1371/journal. pone.0067604.
105. Chen CC, Murray PJ, Jiang TX, et al. Regenerative hair waves in aging mice and extra-follicular modulators Follistatin, Dkk1 and Sfrp4. J Invest Dermatol. 2014;134:2086-2096.
106. Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15:e10.
107. Ahmed MI, Alam M, Emelianov VU, et al. MicroRNA-214 controls skin and hair follicle development by modulat-ing the activity of the Wnt pathway. J Cell Biol. 2014;207:549-567.
108. Zhang GE, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facil-itates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28-41.
109. Harris DA, Patel SH, Gucek M, et al. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One 2015;e0117495. DOI:10.1371/journal.pone.0117495.
111. Li J, Sherman-Baust CA, Tsai-Turton M, et al. Claudin containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244. DOI:10.1186/1471-2407-9-244.
118. Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exo-somes from T cells to antigen-presenting cells. Nature Commun. 2011;2:282. DOI:10.1038/ncomms1285.
119. www.systembio.com [cited 2015 Apr 11].
120. Basu J, Ludlow JW. MSC sourced exosomes as therapeu-tic agents for wound healing and skin regeneration: from scaled production to functional regenerative out-comes in vitro and in vivo. International Society for Stem Cell Research Annual Meeting; 2015 Jun 24-27; Stockholm.
121. Basu J, Ludlow JW. Developmental engineering the kid-ney: leveraging principles of morphogenesis for renal regeneration. Birth Defects Res C Embryo Today. 2012;96:30-38.
122. Genheimer CW, Ilagan RM, Spencer T, et al. Molecular characterization of the regenerative response induced by intrarenal transplantation of selected renal cells in a rodent model of chronic kidney disease. Cells Tissues Organs. 2012;196:374-384.
123. www.fda.gov/BiologicsBloodVaccines/default.htm [cited2015 Apr 11].