DEXON Pharmaceuticals Co., Ltd. (located in Chuo-ku, Tokyo; represented by Shoji Koga) is pleased to announce that it has obtained a patent (Patent No. 7007754) for its ACE2 binding inhibitory composition SGF, effective in preventing viral infections, including COVID-19. In preparation for the potential spread of infections caused by the Omicron variant of the novel coronavirus, DEXON Pharmaceuticals will provide this innovation to medical collaboration institutions (private healthcare facilities).
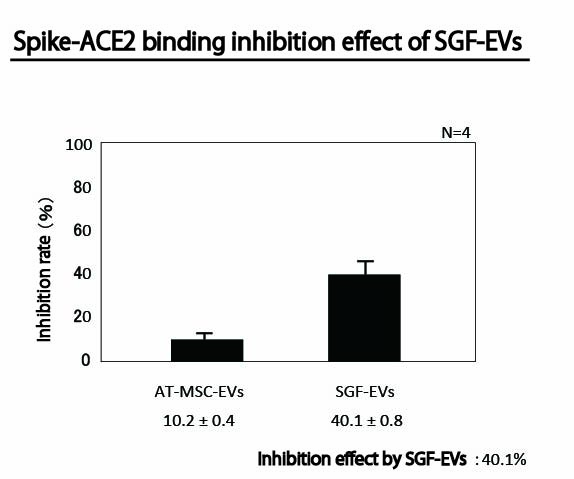
Spike-ACE2 Binding Inhibitory Effect of SGF-EVs
■Mechanism of Action
By inhaling SGF using an inhaler, it inhibits the binding between viruses that utilize ACE2 receptors, such as COVID-19, and ACE2. Specifically, SGF contains exosomes expressing ACE2 derived from deciduous tooth pulp stem cells, and the use of these exosomes can inhibit or block the attachment of SARS-CoV-2 to host cells.
While it was previously reported that it is preferable to use ACE2-negative MSC (mesenchymal stem cells) for COVID-19 treatment, we have discovered that using ACE2-positive compositions can prevent COVID-19 in healthy individuals. This led to the invention of a more effective composition.
■Method of Supply to Medical Institutions
The preventive SGF, which is prepared as an in-house formulation, is manufactured and used in medical collaboration institutions through inhalation or nasal administration using an inhaler.
Due to the nature of in-house formulations, it requires in-person visits to medical collaboration institutions. However, as this medical procedure has an extremely low level of invasiveness on the body, we plan to implement telemedicine and provide SGF and inhalers to the homes of those who wish to receive them, aiming to offer these services nationwide.
■Safety
SGF has been used for elective medical treatments in medical collaboration institutions (Solaria Clinic Group, represented by Shoji Koga) since 2017. As of October 2021, over 2,500 individuals have received treatments involving SGF, and the results indicate that there have been no serious adverse effects.
■Background of the Announcement
■Healthcare Institutions Implementing Preventive Measures
Solaria Clinic Group
Address: 5F Ginza Willow Avenue BLDG, 1-5-8 Ginza, Chuo-ku, Tokyo 104-0061, Japan
TEL : 03-5524-1850
Email: info@ginza-solaria.com
【Company Overview】
Company Name: DEXON Pharmaceuticals Co., Ltd.
Representative: Shoji Koga, Representative Director
Address: 3F Huurick Yaesu-dori Building, 3-5-12 Nihonbashi, Chuo-ku, Tokyo, Japan
Established: April 30, 2020
Capital: 16.5 million yen