The Fourth Cancer Immunotherapy: NKT Cell Targeted Therapy
Description of RIKNKT® using Alpha Galactosylceramide to stimulate self-dendritic cells
This treatment has been approved and deliberated by the Japan Pharmaceutical Law Association’s Certified Regenerative Medicine Committee, based on the “Act on Securing the Safety of Regenerative Medicine and Other Matters” (Act No. 85 of 2013) and the “Ministerial Ordinance No. 140 of the Ministry of Health, Labour and Welfare.” It has been submitted to the Minister of Health, Labour and Welfare for approval and has been accepted.
Name of Regenerative Medicine: NKT Cell Targeted Therapy using Alpha Galactosylceramide to stimulate self-dendritic cells (RIKNKT®) Medical Facility Providing Regenerative Medicine: Solaria Clinic Tokyo Facility Manager: Yoshi Tsuguhisa Koga Person Responsible for Implementation and Medical Doctor Performing Regenerative Medicine: Yoshi Tsuguhisa Koga
- Contents of NKT Cell Targeted Therapy
1) Immune Cell Therapy
Current standard cancer treatments include surgery, chemotherapy, and radiation therapy. However, in cases where these standard treatments are not feasible due to side effects or physical limitations, or in situations of cancer metastasis or recurrence, treatment becomes more challenging. Therefore, efforts to utilize immune cells that can attack cancer for cancer treatment have been ongoing for over 30 years. With rapid advances in immunology, clinical trials of new cancer immunotherapies have been conducted worldwide.
2) Types of Immunotherapy
Cancer immunotherapy includes various approaches such as activation of αβ or γδ T lymphocytes, cancer peptide vaccines, dendritic cell vaccines, NK (Natural Killer) cells, NKT (Natural Killer T) cells, and even genetically modified immune cells. Among these, some have received approvals, some are still in research or clinical trials, and some are offered as private healthcare services in private medical institutions.
3) What is NKT Cell-Targeted Therapy (RIKNKT®)
※GMP Standards: There is a law called “Ministerial Ordinance on Standards for Manufacturing and Quality Control of Drugs and Quasi-Drugs” that specifies the requirements that those involved in pharmaceutical manufacturing must adhere to. These standards for the manufacturing and quality control of pharmaceuticals are referred to as “GMP (Good Manufacturing Practice)” in abbreviation.
Reference: RIKEN Center for Integrative Medical Sciences, RIKEN Institute of Physical and Chemical Research.
4)Indications Malignant tumors and individuals requiring improvement in immune function as determined by a physician.
5)Eligibility Criteria
① Gender: Any
② Age: 16 years and older
③ ECOG Performance Status: Within the range of 0-3
④ Consent:
i. Consent to the contents described in the “Explanation and Consent Form for ‘Alpha-Galactosylceramide-Stimulated Autologous Dendritic Cell Targeted NKT Cell Therapy (RIKNKT®).”
ii. Consent to the contents described in the “Request and Consent Form for Research Use.”
⑤ Exclusion Criteria and Conditions: Determined based on medical history, examinations, etc.
i. Patients with T-cell or NK-cell type malignant lymphoma or leukemia who do not consent to receiving the same treatment.
ii. Individuals with severe autoimmune diseases (such as psoriasis) as determined by a physician to be ineligible.
iii. Active bronchial asthma.
iv. Bacterial infections (diagnosed based on symptoms such as fever and increased white blood cell count).
v. Individuals with a history of bone marrow or organ transplantation.
vi. Pregnant or potentially pregnant women, as well as women in the lactation period.
vii. Carriers of virus infections such as HIV, HTLV-1, or individuals suspected of syphilis infection.
viii. Serious heart diseases, lung diseases, liver diseases, infectious diseases, and other complications, as well as diseases and conditions that a physician comprehensively considers to be unsuitable. Specific numerical values for unsuitability are as follows:
- White blood cell count: 10,000/uL or higher
- Hemoglobin: Less than 9.0g/dL
- Platelet count: Less than 100,000/mL
- Blood pressure: Systolic blood pressure of 200mmHg or higher or diastolic blood pressure of 80mmHg or lower
- SpO2 (room air): Less than 94%
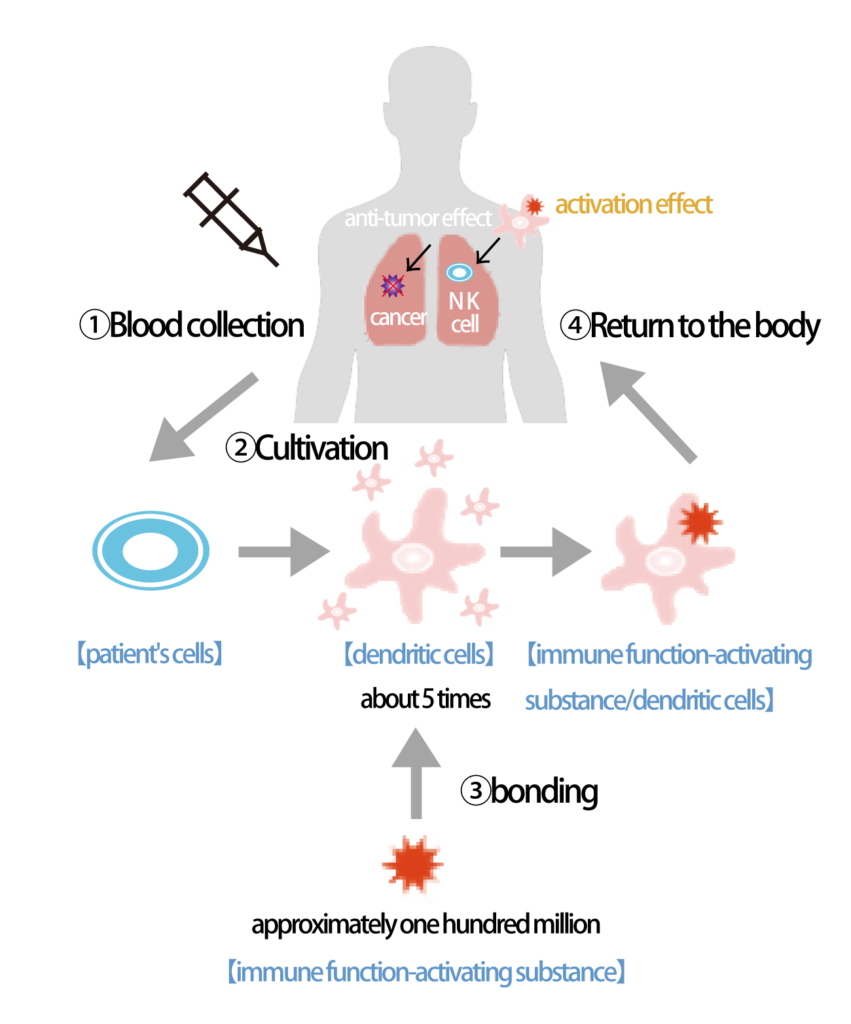
6)Procedure from Blood Collection to Cell Infusion
① Conduct a medical interview, examination, and infectious disease testing. The results of infectious disease testing will only be communicated to the patient and individuals specified by the patient. If the tests for HIV, adult T-cell leukemia virus, or syphilis are positive, NKT cell-targeted therapy may not be administered due to the possibility of worsening infections or the priority of infection treatment.
List of Examinations
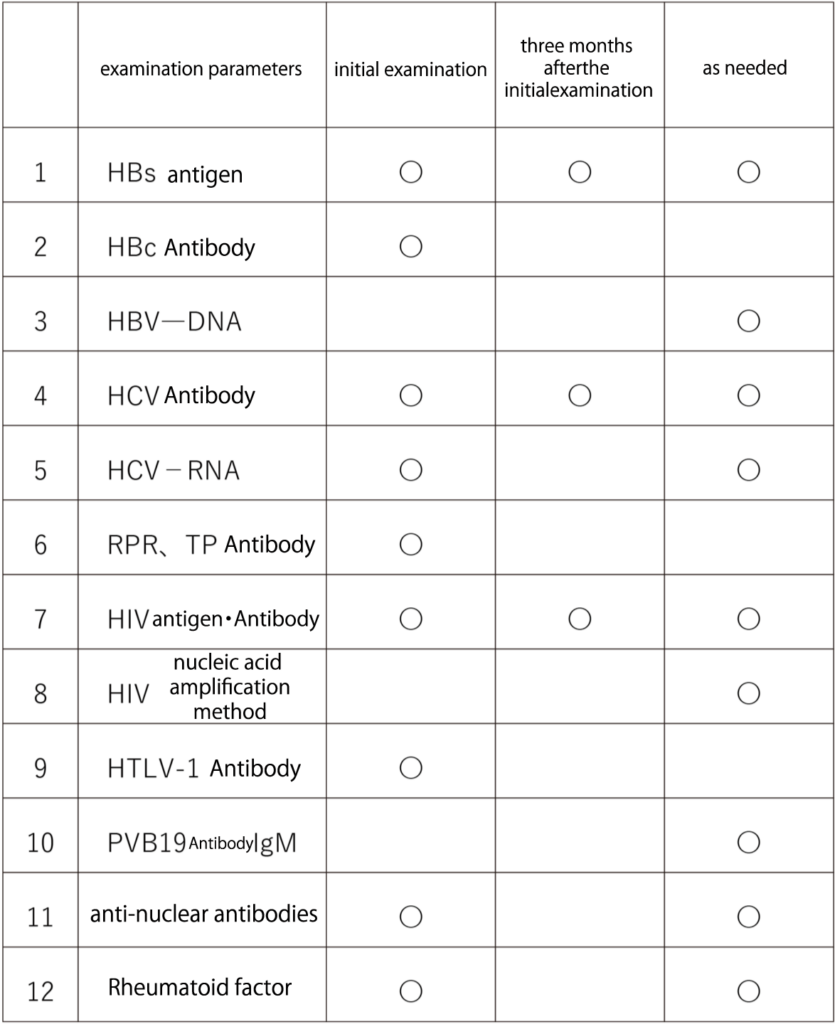
The initial examination is a prerequisite test required for receiving cell therapy. The examination conducted three months after the initial examination is aimed at addressing the “window period” during which viruses may not be detectable immediately after infection. It is advisable to undergo this examination three months later whenever possible. Additionally, supplementary tests are conducted as clinically necessary, such as after receiving a blood transfusion.
②If it is determined based on the results of step ① that treatment is feasible, a method called apheresis is used to collect a large quantity of monocytes from the blood. Apheresis is necessary because monocytes, which are the precursors of dendritic cells, are present in the blood at a ratio of less than 1/1000 lymphocytes. This procedure involves using specialized medical equipment for apheresis, and it circulates a volume of blood externally, aiming to collect approximately 4000 mL of monocytes. The collection process typically takes about 2 to 3 hours. Specifically, a needle is inserted into the veins of the arm or groin, and this is connected to the apheresis machine, which separates monocytes while returning the rest of the blood back into the bloodstream. In rare cases, if the physician determines that apheresis cannot be performed through the veins due to the condition of the patient’s blood vessels, apheresis may be carried out through an artery.
③ After isolating monocytes from the blood, they are differentiated into dendritic cells and matured through sufficient time of cultivation. Subsequently, they are stimulated with “GMP-compliant alpha-galactosylceramide.” Cell cultivation is outsourced to the BioAxel Co., Ltd. Kyoto Katsura Venture Plaza Cell Culture Processing Facility.
④ Following the completion of cultivation, dendritic cells that have been stimulated with “GMP-compliant alpha-galactosylceramide” are frozen for storage.
⑤ To confirm the safety of the frozen dendritic cells, various infection tests, including sterile tests, are conducted over the course of one week.
⑥ After confirming safety in step ⑤, the dendritic cells that were stimulated with “GMP-compliant alpha-galactosylceramide” and kept in frozen storage are transferred to our clinic under conditions of -20°C and are subsequently stored at -80°C at our clinic. When the recipient visits the clinic, the standard protocol is to administer the treatment in four separate doses every two weeks. However, this may vary if the physician deems it appropriate and the patient consents. Administration is carried out through intravenous infusion, typically mixed with approximately 50 mL of normal saline solution.
7) Ensuring the Safety of Administered Cells The cultivation of dendritic cells is conducted under the strict technical management of the BioAxcel Co., Ltd. Kyoto-Katsura Venture Plaza Cell Culture Processing Facility. However, it is of utmost importance to conduct multiple checks before administration to confirm that the cultured cells are free from contamination, such as bacteria. To ensure the safety of cell administration, various tests are conducted before administration, including sterility testing, mycoplasma testing, endotoxin testing, and other measures.
8) If the Cultured Dendritic Cell Count Does Not Meet Specifications The cultivation of dendritic cells is carried out in a cleanroom under the supervision of advanced technical management. However, the characteristics and quantity of obtained dendritic cells may vary depending on the condition of the collected blood. (In cases where an adequate supply of dendritic cells cannot be ensured or if they do not meet the required standards, treatment cannot proceed as scheduled.)
9) Supply and Storage of Blood and Cultured Cell Samples For the purpose of treatment and as a reference in case of infections or other unexpected events, it is mandatory to retain a very small portion of the patient’s blood and cultured cells for a certain period. We kindly request a partial contribution of your blood and cultured cells. The quantity of samples to be retained is minimal (approximately 1mL) and will not impact the treatment in any significant way.
The provided samples will be stored and managed at the BioAxel Co., Ltd. Kyoto Katsura Venture Plaza Cell Culture Processing Facility for a period of one year. These samples will be used for investigating the causes in case of occurrences such as diseases during or after the treatment. For the purpose of cause investigation, the samples will be stored in a frozen state. If not used, they will be appropriately disposed of one year after the start of storage. The provided samples will be used only for the disclosed purposes and will not be used for the treatment of others.
- Expected Effects and Side Effects
1) About the Effects
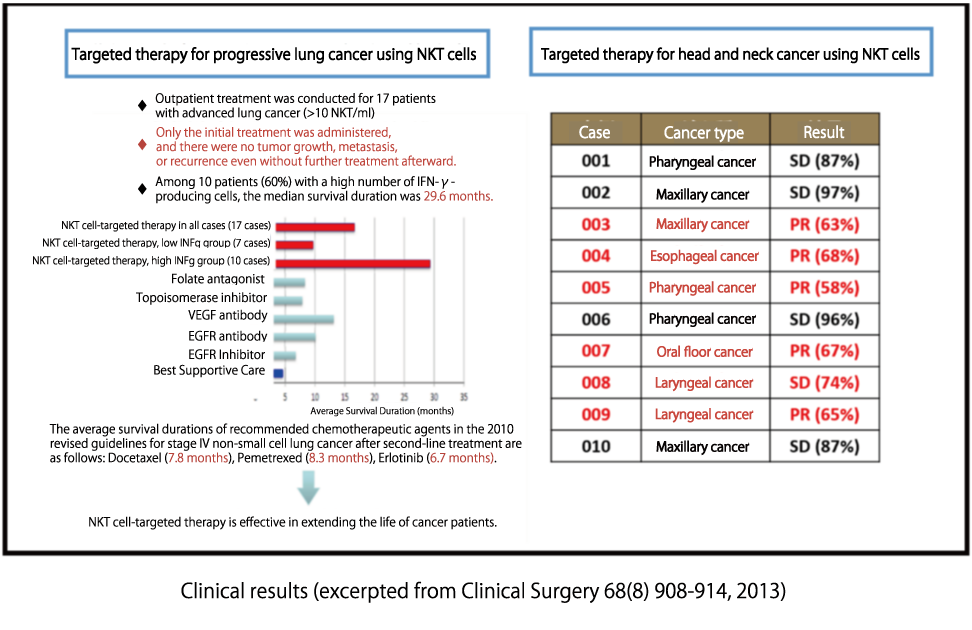
NKT cell-targeted therapy has already undergone clinical trials at universities and research institutions, with results suggesting significant efficacy in advanced lung cancer and head and neck cancer. However, it’s important to note that the effects of the treatment can vary from person to person depending on factors such as the patient’s condition, disease state, and blood profile. Please be aware of this individual variability in treatment outcomes.At our clinic, we seek the cooperation of patients who are currently undergoing treatment at other hospitals to collect necessary test data for evaluating treatment efficacy. We are committed to providing each patient with the most suitable administration method to ensure reliable and effective treatment. While NKT cell-targeted therapy theoretically has the potential to benefit all cancer patients, it is important to emphasize that the treatment results have not been verified for all cancer patients.For reference, we present the results of physician-led trials conducted for NKT cell-targeted therapy in lung cancer and head and neck cancer.
2) Regarding Treatment Side Effects
After administering dendritic cells stimulated with『GMP-compliant alpha-galactosylceramide』, some patients may experience mild fever and fatigue. In most cases, these symptoms are minor, with fever reaching around 38°C and resolving within two days. Additionally, very rarely, there may be symptoms that appear to be indicative of allergic reactions. We conduct treatment with careful observation, and in the event of such side effects, we respond promptly and appropriately.
3) Regarding Apheresis Side Effects
During apheresis, blood is circulated outside the body through veins in the arm, groin, etc. The puncture of the blood vessels is performed by a proficient physician with thorough disinfection. Occasionally, subcutaneous bleeding may be observed. Additionally, there might be symptoms of dizziness, nausea, and even a drop in blood pressure due to anxiety about the blood collection. We manage these symptoms by observing and adjusting the collection rate.
Apheresis involves the use of anticoagulants to prevent blood from clotting, but the citrate contained in these anticoagulants can lead to a decrease in blood calcium levels. As a result, initial symptoms such as numbness of the lips and fingers may occur, and as it progresses, nausea, vomiting, and muscle twitching of the fingers may develop. If initial symptoms are observed, we slow down the collection/return rate at that stage or administer medication to correct calcium levels for symptom improvement.
Sometimes, a decrease in platelet count may be observed, but it usually recovers quickly, and platelet transfusion is not typically required.
- Availability of Other Treatment Options, Their Contents, and Comparison with the Effects and Side Effects of Other Treatments
1) Other Treatment Options
There are three standard cancer treatments available, including surgical procedures, chemotherapy, and radiation therapy. Additionally, there have been recent advancements in antibody-based therapies. These treatments have already been established as effective methods for cancer treatment. If you are currently undergoing or planning to undergo any of these treatments, we will determine the optimal timing and intervals for administering the ‘GMP-compliant alpha-galactosylceramide-stimulated dendritic cells’ based on your treatment schedule whenever possible. - Comparison with the Effects of Other Treatment Options
Surgical procedures, radiation therapy, and chemotherapy have been the standard treatments for many years both domestically and internationally. Clinical research and applications have been conducted, and scientific and medical evidence has been presented regarding their effectiveness in terms of tumor reduction and prolonging life.
NKT cell-targeted therapy (RIKNKT®), developed by RIKEN Immunotherapy Corporation, is currently provided legally under the “Act on the Safety of Regenerative Medicine and Other Therapies” (Act No. 85 of 2013), as established by the Ministry of Health, Labour and Welfare.
- Comparison with side effects of other treatments
The content, severity, and frequency of side effects of standard treatments are well documented. These side effects range from mild to severe and include symptoms such as loss of appetite, diarrhea, hair loss, skin disorders, peripheral nerve damage, and bone marrow suppression, among others. In our treatment, such side effects are rarely observed. - Post-treatment Follow-up Investigation
Under the “Act on the Safety of Regenerative Medicine” (Act No. 85 of 2013), there are regulations for a certain period of post-treatment follow-up investigation, which includes monitoring the patient’s condition and any occurrence of diseases. To track the presence of post-treatment side effects and assess the treatment’s effectiveness, we will send you a follow-up survey form from our clinic for a duration of 5 years after the completion of NKT cell-targeted therapy (RIKNKT®). Your cooperation is greatly appreciated. - Freedom to Decline
Even if you have agreed to this treatment, you have the right to withdraw your consent and discontinue treatment at any time, both before and after treatment initiation, regardless of whether it’s before or after cell culture or blood collection for cultivation. Your decision to withdraw consent will not result in any disadvantages for your subsequent treatment. However, please note that there may be charges associated with cell culture expenses if withdrawal occurs after the start of cell cultivation. - Protection of Personal Information
We will protect personal information in accordance with the clinic’s privacy policy. Personal information, such as names that can identify patients, will not be provided or disclosed to others without the patient’s consent.Personal information, including names, will be managed rigorously through information sharing between related facilities, such as BioAxel Co., Ltd. Kyoto Kei Venture Plaza Cell Culture and Processing Facility, which is responsible for cell culture outsourcing, and RIKEN Immunotherapy Medicine Corporation, which manages the treatment process in cooperation with the provider of “GMP-compliant alpha-galactosylceramide.” - Costs Associated with the Procedure
Initial consultation fee: ¥30,000 (excluding tax)
Infectious disease testing fee (only for the initial consultation): ¥30,000 (excluding tax)
Treatment fee: ¥3,145,000 (excluding tax)
*Regardless of the reason, if cell culture has been initiated after the start, charges for the culture expenses incurred up to that point will apply.
*Our clinic operates as private medical care (not covered by public medical insurance).
- Compensation for Health Damage
In the event that a patient experiences health damage due to blood collection or treatment, the severity of the damage will determine the appropriate response. If it can be managed within the clinic’s capabilities, suitable treatment will be provided. In cases where handling is difficult, the patient may be referred to a hospital where admission is possible. Compensation for health damage deemed to be due to negligence will be handled through medical malpractice liability insurance covering the physician and the medical facility. - Ownership of Patent Rights, Copyrights, Property Rights, and Economic Interests
As a result of the treatment, there may be patent rights or economic interests related to the treatment. However, these are based on the knowledge obtained from the entire provided sample, and individual patients do not have ownership of patent rights, copyrights, property rights, or economic interests. - Freedom to Ask Questions
If you have any questions or complaints regarding the provision, storage, or research related to this treatment, please feel free to ask the physicians at Solaria Clinic Tokyo
(Open from 11:00 AM to 6:00 PM, closed on Sundays).
- About the Certification Committee for Regenerative Medicine, etc.
The NKT cell-targeted therapy using Alpha-Galactosylceramide-stimulated autologous dendritic cells provided at Solaria Clinic Tokyo (RIKNKT®) has undergone evaluation by the Japan Society for Regulatory Science’s Committee for Regenerative Medicine, etc.
Japan Society for Regulatory Science’s Committee for Regenerative Medicine, etc. (Certification Number: NB3140007) Contact Information: Address: Ogura Building 6F, 8-18-11 Ginza, Chuo-ku, Tokyo, Japan
Phone Number: 03-6264-3883
Email: info@japal.org
Responsible Person: Dr. Shoji Koga
Phone Number: 03-6665-6377 FAX: 03-6665-6378