What is fibroblast therapy?
This enhances the ability to produce collagen and hyaluronic acid. Fibroblasts are essential cells for maintaining the youthfulness of the skin. Our skin is divided into three layers: the epidermis, dermis, and subcutaneous tissue, and fibroblasts are found in the dermis layer. The majority of the dermis is composed of components produced by fibroblasts, which are the three major elements that make up the skin: collagen, elastin, and hyaluronic acid.
Collagen is a protein fiber that is intricately woven in a mesh pattern to support the skin’s structure and firmness. Elastin binds collagen together and contributes to the skin’s elasticity. Hyaluronic acid is a gel-like substance that fills the gaps in the dermis, stabilizing its structure and influencing skin hydration.
Furthermore, fibroblasts play roles in maintaining vascular health, producing female hormones (estrogen) related to beautiful skin, and moving to damaged tissue when the skin is injured to increase collagen production and assist in tissue repair. If fibroblasts are active, they contribute to keeping the skin healthy. However, fibroblasts can weaken and decrease due to various factors, with aging being the most prominent, but other factors like UV radiation, dryness, and stress also contribute. As a result, the production of beauty-related components decreases, leading to skin aging such as wrinkles and sagging.
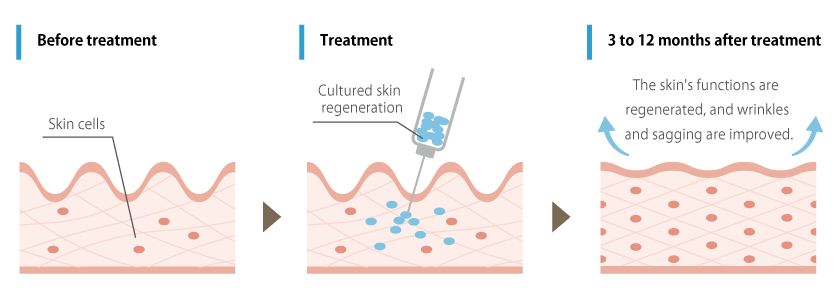
To address such skin aging concerns, our clinic offers a treatment using autologous fibroblasts. This treatment involves harvesting the patient’s own skin cells (fibroblasts), cultivating and proliferating them at our affiliated CPC (Cell Processing Center), and then transplanting them to the targeted areas of concern. By enhancing fibroblasts that have aged or decreased due to factors like aging and UV damage, we aim to activate their function and promote skin regeneration.
The skin cells are collected from behind the ear, so there are no noticeable marks after the procedure. Currently, treatments for wrinkles and skin aging often involve the injection of collagen and hyaluronic acid, but there have been concerns about the potential for unknown infections and reports of allergies. In contrast, fibroblast therapy utilizes the patient’s own skin cells, making it virtually free of side effects. Furthermore, it boasts the advantage of long-lasting effects.

For individuals who
- Want to fundamentally improve skin texture.
- Are concerned about wrinkles, sagging, and under-eye circles.
- Wish to delay skin aging.
- Have not been satisfied with conventional treatments.
- Are resistant to treatments involving surgery or foreign substances.
Individuals eligible for treatment
- Those diagnosed with atherosclerotic lesions.
- Those with plaques (IMT1.1 or above) in the carotid arteries (common carotid artery, internal carotid artery) based on carotid artery ultrasound.
- Those with a history of myocardial infarction or angina pectoris.
- Those showing irregularities, calcification, stenosis, or occlusions in coronary artery CT scans.
- Those with a history of stroke.
- Those with evidence of cerebral infarcts in head MRI or MRA scans.
- Those with two or more second-degree blood relatives who have atherosclerotic diseases.
- Those determined to have a high genetic predisposition for atherosclerosis through genetic testing.
Individuals ineligible for treatment
1)Those under 18 years of age at the time of treatment application.
2)Those not in a health condition sufficient to tolerate fat extraction.
3)Those lacking the capacity for informed consent or unable to obtain consent from a legal representative.
4)Those who have not accepted the informed consent document for this treatment, received adequate explanation, and provided voluntary written consent (if a legal representative has not consented in writing).
5)Individuals determined by the attending physician as not suitable for treatment based on medical history, examinations, or other factors.
6)Pregnant individuals.
7)Individuals currently undergoing treatment for gynecological disorders.
8)Those diagnosed with proliferative diabetic retinopathy or age-related macular degeneration.
9)Individuals with poorly controlled hypertension or cardiac arrhythmias.
10)Those exhibiting clinical symptoms of delirium.
11)Those with a known hypersensitivity to penicillin.
12)Individuals currently undergoing treatment for or hospitalized within the past 3 months due to cerebrovascular disorders such as stroke, intracerebral hemorrhage, or subarachnoid hemorrhage.
13)Individuals currently undergoing treatment for brain tumors or suffering from untreated depression or depression unresponsive to treatment.
14)Those with positive results from infection tests such as hepatitis B, hepatitis C, HIV, syphilis, within the last 12 weeks.
The exclusion criteria include individuals who cannot make decisions for themselves, those unable to attend follow-up appointments, individuals with a fear of treatment, those with impaired cardiac and pulmonary function, those with weakened immune function, and those with allergies, among others.
Treatment Procedure
STEP1
Counseling
After receiving sufficient explanation, you will be asked to submit a consent form based on the treatment plan.

↓
STEP2
Blood Test and Blood Collection
For the collection of your own cells, a local anesthetic will be administered, and a sample will be taken from the oral mucosa gingival buccal transition area (the mucous membrane at the border between the gums and the cheek) with a diameter of approximately 3.5 mm. After the collection, it will be sutured with one absorbable thread. On the day of cell collection, 50 ml of blood will be drawn to prepare the culture medium. Additionally, blood for the culture medium will be collected as needed during subsequent visits after the first injection treatment. Following the first blood collection, additional 50 ml blood samples may be taken approximately 2 to 4 times as needed. In cases where cell proliferation is poor, there is a possibility that tissue collection may be requested again. (Out of 98 initial cases, repeat collections were performed in 5 cases, including consultations with related institutions, and a second repeat collection was performed in one of these cases.)
↓
STEP3
Cultivation
The fibroblasts obtained from the mucous membrane are cultured in a culture medium containing autologous blood components for approximately two months.
↓
STEP4
Administration
A highly concentrated cell suspension prepared through cultivation is created, and from approximately two months after mucous membrane collection, autologous fibroblasts cultured in the wrinkled skin are injected into the wrinkled areas 2-3 times, with each injection being 1-4 ml. If any abnormalities are observed, the procedure will be immediately halted.
↓
STEP5
Follow-up (Observation of Progress)
After the injection, evaluations of the treatment will be conducted at 3, 6, 9, and 12 months post-treatment. The evaluation will involve diagnostic assessments through photography before and after the procedure, assessing the satisfaction of the patient, and evaluations by the physician. If any issues arise during the post-injection observation period or after one year, we kindly request that you contact us by phone for consultation or visit our clinic in person.
Expected Effects and Possible Side Effects
Expected Effects
The injected autologous cultured fibroblasts are expected to produce extracellular matrix components such as collagen, leading to skin improvement through the reconstruction of subcutaneous tissue. Previous research has shown that quantitative analysis one year after treatment has demonstrated significant increases in skin hydration, brightness, and fine texture, which typically decline with age.
Possible Side Effects
There can be individual variations in treatment effectiveness, and some individuals may not perceive the expected effects.(Out of the 45 cases for which statistics were available, 3 individuals reported being “very dissatisfied,” and 4 individuals reported being “dissatisfied” in a subjective 5-point evaluation conducted one year after treatment.)
【Minor side effects may include】
Pain during local anesthesia injection for mucous membrane collection.Discomfort at the collection site after the procedure (due to the wound or sutures, lasting about one week)Pain during fibroblast injection.Temporary redness after injection.Repeated tissue collection in cases where cell proliferation is insufficient.
【Severe complications】
Prolonged redness and swelling after injection.Infections.Allergic reactions.Formation of tumors※.Pigmentation issues.(Among 150 injections evaluated in treatment cases at affiliated medical institutions related to Nagoya University, prolonged redness was observed only once after injection but resolved within approximately two weeks (transient redness was observed on the day of injection and the following day). No other issues were reported. Additionally, clinical studies similar to this treatment conducted at Nagoya University have observed immediate redness after injection but have not reported any adverse events.)
※Tumor formation… While it has not been observed in this treatment, including affiliated facilities, there have been reports of tumor formation in methods similar to this treatment, which involve centrifugation of blood and injection into the subcutaneous tissue, particularly in methods that involve the addition of growth factors.
Precautions for This Treatment
On the day of administration, please refrain from strenuous exercise, staying up all night, excessive alcohol consumption, and similar activities.
Treatment Costs
This treatment is entirely self-pay, and it cannot be covered by health insurance.
Storage and Disposal of Samples
A portion of the collected and cultured cell preparations will be stored at -80 degrees Celsius for a minimum of 10 years before each treatment. The blood collected during this treatment will only be used for your own treatment. If treatment results are used as research data, separate confirmation of your or your legal representative’s consent for usage will be obtained. Furthermore, any information that could identify you or your personal details, including the disclosure of your name, will not be publicly disclosed, including in the presentation of treatment outcomes.
Response to Significant Insights on Health, Genetic Characteristics, etc.
As this treatment is not conducted as research, compensation for health damage is not mandatory. However, if health damage occurs as a result of this treatment, necessary measures will be taken.
Evaluation and Notification of this Treatment
Various Application Form Support Sites https://saiseiiryo.mhlw.go.jp
In accordance with the Act on the Safety of Regenerative Medicine, when conducting treatment using fibroblasts at our clinic, we have submitted a regenerative medicine plan to the Minister of Health, Labour and Welfare after seeking the opinions of the Regenerative Medicine and Related Measures Committee. Furthermore, our clinic’s completion of the submission of the regenerative medicine plan has been publicly disclosed on the Ministry of Health, Labour and Welfare’s website under “Various Application Form Support Sites.”
Committee that conducted the review: Tokyo Edogawa Designated Regenerative Medicine Committee.