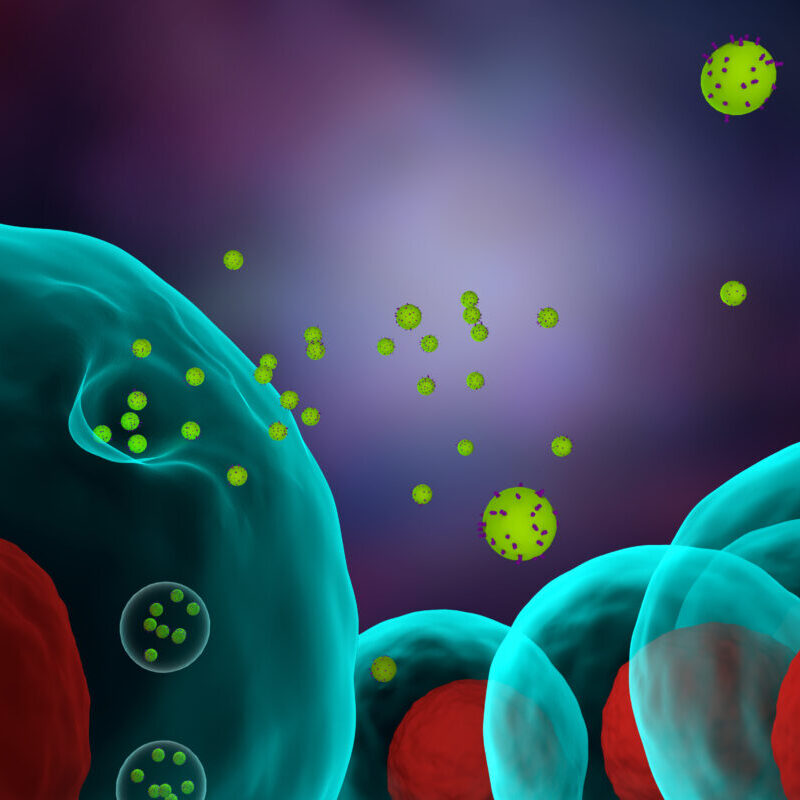
What is Osteoarthritis of the knee joint
Osteoarthritis of the knee joint is a condition characterized by pain in the knee due to changes in the structure of the knee bones and degeneration of the joint cartilage.
Aging, obesity, repetitive activities that place excessive stress on the knee joint, and the sequelae of trauma (such as fractures, ligament injuries, meniscus injuries, etc.) are among the causes. The stress on the knee gradually wears down the cartilage, leading to inflammation. Pain occurs, especially during the first few steps when walking or when getting up from a chair.
As the disease progresses, fluid may accumulate in the knee, making it difficult to bend and extend the knee. Symptoms such as sitting in the Japanese seiza or cross-legged position become challenging, and going up and down stairs can be difficult. In the advanced stages, the bone itself can be damaged, leading to deformities like bowlegs (genu varum) or knock-knees (genu valgum), and the legs may become immobile, making even walking extremely painful.
In such a condition, physical activity decreases, and there is a risk of a significant decline in physical fitness. In the worst-case scenario, it may lead to being bedridden.
Damage to the cartilage and meniscus caused by osteoarthritis of the knee joint cannot be completely reversed with standard treatments. Therefore, symptomatic treatments such as oral pain relievers, intra-articular injections of hyaluronic acid, arthroscopic surgery, knee realignment procedures, and in more advanced cases, knee replacement surgery (total knee arthroplasty) may be necessary. It’s important to note that knee replacement surgery, in particular, has its drawbacks, including potential complications and limitations in daily life and sports activities.
The autologous adipose-derived mesenchymal stem cell therapy offered at our clinic holds the potential for knee cartilage regeneration through the use of stem cells. There is no need to continue pain relievers or hyaluronic acid injections, nor is complex surgery necessary. At our clinic, we provide treatment through regenerative medicine using autologous adipose-derived mesenchymal stem cells. This involves isolating stem cells from a very small amount (4-5 cubic centimeters) of one’s own adipose tissue, culturing and expanding them to approximately 100 million cells at our affiliated CPC, and then administering them through methods such as intravenous drip or injection. This treatment method is minimally invasive.
Patients eligible for treatment.
・Experience knee pain after walking for an extended period
・Find it difficult to bend or straighten the knee
・Struggle with sitting cross-legged or in the seiza position
・Have difficulty going up and down stairs
・Prefer non-invasive treatments without the need for hospitalization or surgery
For more details, please inquire by phone or email.
Benefits of Adipose Stem Cell Therapy
1.”Using one’s own adipose-derived stem cells results in fewer side effects and ensures safety.”
You can undergo treatment using your own adipose-derived stem cells.”

2.No Hospitalization or Surgery Required
Adipose stem cell therapy can be performed on an outpatient basis, both for the collection of adipose tissue and the administration of autologous adipose-derived stem cells. It is a low-impact treatment with minimal physical burden.
3.Lifetime Treatment Possibility Through Stem Cell Storage
Cultured stem cells can be preserved for future use, allowing for additional injections as needed.
Treatment Process
Treatment can be done on an outpatient basis, and there is no need for hospitalization.
STEP01
Counseling
Explanation and consent are exchanged in writing.

↓
STEP02
Blood Tests
Pre-tests and suitability assessments are conducted.
↓
STEP03
Fat Harvesting and Blood Collection
A small amount of fat is harvested from a location, typically the abdomen, where fat can be reliably obtained under local anesthesia.
Blood collection of 100ml is performed for cultivation purposes.
↓
STEP04
Cell Cultivation and Proliferation at the CPC (Cultivation Facility)
Cultivation is carried out for approximately 6-8 weeks, aiming to culture approximately 100 million cells.
There may be individual variations in the rate of cell growth.

↓
STEP05
Injecting into the affected area
The cultured stem cells will be injected into the knee joint.
↓
STEP06
Follow-up Observation
We aim to observe patients as closely as possible at 1, 3, 6, and 12 months after stem cell administration.
Expected Benefits and Potential Side Effects
Expected Benefits
Adipose-derived mesenchymal stem cells have the ability to differentiate (acquire morphology and function) into nerve, fat, muscle, bone, cartilage, and other internal organ tissues. This capability makes it possible to repair damaged or aging cells.
Furthermore, the paracrine effects of liquid factors such as cytokines and exosomes produced by stem cells can play a role in immune system regulation, angiogenesis, anti-inflammatory effects, antioxidant effects, anti-apoptotic effects, tissue repair, and various therapeutic benefits can be expected.
Potential Side Effects
【During Fat Tissue Harvesting】
・Bleeding from the incision site.
・Infection or pain at the incision site.
・ide effects related to anesthesia medication, allergic reactions (palpitations, hives, difficulty breathing, etc.).
【Side Effects during Administration】
・Fever after stem cell administration
Typically resolves within 24 hours.
・Allergic reactions
Reports of allergic symptoms such as palpitations, hives, and difficulty breathing during treatment.
・Unexpected adverse events
There have been cases in the past where patients who received adipose-derived mesenchymal stem cell therapy died from pulmonary embolism. The causal relationship between stem cell administration and death due to pulmonary embolism is unclear, but pulmonary embolism is the most dangerous complication of intravenous stem cell administration.
In the event that pulmonary embolism occurs during the course of treatment, our clinic will respond as follows.
①If it occurs within the clinic, we will assess the severity based on the guidelines for pulmonary thromboembolism treatment, promptly implement respiratory and circulatory management, and arrange for emergency transportation to affiliated medical institutions, and so on.
②If you experience sudden symptoms such as difficulty breathing, chest pain, or cold sweats outside the clinic, please contact our clinic by phone. We will assess whether an ambulance is required and provide information about affiliated medical institutions, if necessary.
③Regarding unexpected adverse events, we will initially handle them at our clinic. Therefore, if you experience any distressing symptoms accompanied by pain after stem cell administration, please contact our clinic (or the emergency contact line outside of regular business hours). In the unlikely event that we are unable to address the symptoms at our clinic, we will provide guidance to external medical institutions with inpatient facilities.
Important Considerations for This Treatment
On the day of administration, please refrain from intense physical activity, staying up all night, excessive alcohol consumption, and the like.
Sample Storage and Disposal Methods
The blood collected in this treatment will be used exclusively for your own treatment. In cases where treatment results are used as research data, separate confirmation of consent for use will be obtained from you or your surrogate decision-maker. Furthermore, any information that can identify you or your personal details, including the announcement of treatment results, will not be disclosed.
In case of Cultivation Failure
During fat tissue harvesting or adipose-derived mesenchymal stem cell cultivation, there is an extremely rare chance of contamination, such as bacteria or fungi. If contamination (referred to as “contamination”) is confirmed, all cultured cells are discarded, and administration cannot proceed. In such cases, the material will be disposed of. If you wish to continue treatment, you will need to undergo fat tissue harvesting again at no additional cost.
Review and Notification of This Treatment
In accordance with the Act on the Safety of Regenerative Medicine and other related laws, we have obtained approval for “appropriate” in the review by the Committee on Regenerative Medicine and others before submitting the Regenerative Medicine Provision Plan to the Minister of Health, Labor, and Welfare for treatment using autologous adipose-derived stem cells at our clinic.
If you wish to review the plan, please contact our inquiry desk.
Frequently Asked Questions(FAQs)
Q: Is hospitalization required?
A: While two visits are necessary, one for the collection of the patient’s own tissues that serve as the source of the administered cells and another after cultivation is completed for the actual administration, both procedures can be done as outpatient treatments.
Q: How long does the treatment take?
A: The collection of the patient’s own tissues takes about 1 hour, and the treatment itself via intravenous drip typically takes around 1 to 2 hours to complete. Since we operate on a fully scheduled appointment system, there is no waiting time.
However, please note that the cultivation of adipose stem cells will take approximately 6 to 8 weeks. Therefore, the overall timeframe from the initial consultation to the treatment is approximately 2 to 2.5 months.
Q: How much of my own tissue is needed for the treatment?
A: Approximately 3-5 cubic centimeters of tissue will be collected. Local anesthesia is administered during the collection, so there is very little pain involved.
Q: Is safety ensured?
A: Treatment through regenerative medicine requires the submission of a treatment provision plan to the Ministry of Health, Labor, and Welfare and cannot be provided without acceptance. Our clinic’s application has been accepted, and we offer a treatment method that has undergone strict safety evaluations domestically.
Q: Is it eligible for medical expense deduction?
A: If it is for medical treatment purposes, it may be eligible for medical expense deduction. For detailed procedures and information, please inquire at your nearest tax office.
On the day of administration, please refrain from intense physical activity, staying up all night, excessive alcohol consumption, and the like.
Sample Storage and Disposal Methods
The blood collected in this treatment will be used exclusively for your own treatment. In cases where treatment results are used as research data, separate confirmation of consent for use will be obtained from you or your surrogate decision-maker. Furthermore, any information that can identify you or your personal details, including the announcement of treatment results, will not be disclosed.
In case of Cultivation Failure
During fat tissue harvesting or adipose-derived mesenchymal stem cell cultivation, there is an extremely rare chance of contamination, such as bacteria or fungi. If contamination (referred to as “contamination”) is confirmed, all cultured cells are discarded, and administration cannot proceed. In such cases, the material will be disposed of. If you wish to continue treatment, you will need to undergo fat tissue harvesting again at no additional cost.
Review and Notification of This Treatment
During fat tissue harvesting or adipose-derived mesenchymal stem cell cultivation, there is an extremely rare chance of contamination, such as bacteria or fungi. If contamination (referred to as “contamination”) is confirmed, all cultured cells are discarded, and administration cannot proceed. In such cases, the material will be disposed of. If you wish to continue treatment, you will need to undergo fat tissue harvesting again at no additional cost.
Comparison with Other Treatment Methods
In accordance with the Act on the Safety of Regenerative Medicine and other related laws, we have obtained approval for “appropriate” in the review by the Committee on Regenerative Medicine and others before submitting the Regenerative Medicine Provision Plan to the Minister of Health, Labor, and Welfare for treatment using autologous adipose-derived stem cells at our clinic.
If you wish to review the plan, please contact our inquiry desk.
Comparison with Other Treatment Methods
Male menopausal syndrome is medically referred to as Late-Onset Hypogonadism Syndrome (LOH syndrome). The symptoms caused by a decrease in testosterone levels often manifest gradually. Despite being characteristic of LOH syndrome, these symptoms are sometimes misdiagnosed as mental disorders like “depression” or “autonomic nervous system dysfunction,” or simply attributed to the effects of aging (“getting older”). As a result, appropriate treatment is not always administered.
The treatment methods employed thus far include hormone replacement therapy, traditional Chinese medicine therapy, ED medications, psychotropic drugs and sleep inducers, medication for lifestyle diseases like hypertension and lipid disorders, and supplement therapy. However, none of these methods are considered fundamental treatments.
Frequently Asked Questions (FAQs)
Q: Is hospitalization required?
A: While two visits are necessary, one for the collection of the patient’s own tissues that serve as the source of the administered cells and another after cultivation is completed for the actual administration, both procedures can be done as outpatient treatments.
Q: How long does the treatment take?
A: The collection of the patient’s own tissues takes about 1 hour, and the treatment itself via intravenous drip typically takes around 1 to 2 hours to complete. Since we operate on a fully scheduled appointment system, there is no waiting time.
However, please note that the cultivation of adipose stem cells will take approximately 6 to 8 weeks. Therefore, the overall timeframe from the initial consultation to the treatment is approximately 2 to 2.5 months.
Q: How much of my own tissue is needed for the treatment?
A: Approximately 3-5 cubic centimeters of tissue will be collected. Local anesthesia is administered during the collection, so there is very little pain involved.
Q: Is safety ensured?
A: Treatment through regenerative medicine requires the submission of a treatment provision plan to the Ministry of Health, Labor, and Welfare and cannot be provided without acceptance. Our clinic’s application has been accepted, and we offer a treatment method that has undergone strict safety evaluations domestically.
Q: Is it eligible for medical expense deduction?
A: If it is for medical treatment purposes, it may be eligible for medical expense deduction. For detailed procedures and information, please inquire at your nearest tax office.